
A 28-year-old man presented in general practice with a three weeks history of acute on chronic right knee pain. He had struggled with right anterior knee pain for 15 years, but managed this with load management and avoiding provocative activity. In the last 3 weeks, he has been partaking in a running event where he has been attempting to run 5km every day.
He denies any previous traumatic event or swelling of the joint. The pain appears to gradually improve during running, but is very painful after exercise. There is some morning soreness/stiffness, but this resolves within 30 minutes. The patient describes the pain as being just below his patella.
He reports no weight loss, night sweats or concerning features. He is normally fit and well and does not take any regular medication.
On clinical examination, there is no redness, swelling or heat to the area. There is no effusion clinically to palpation around the knee joint. He is very tender to palpation of the tendon just below his patella, however, no joint line tenderness. He has full range of movement, but describes pain in deep flexion of the knee. Ligaments of the knee are intact.
Functionally he has normal gait, is uncomfortable in squatting and has no obvious biomechanical misalignment on inspection.
The likely differential diagnoses for anterior knee pain are patella tendinopathy, bursal pathology, Hoffa’s fat pad syndrome, chondromalacia patella or osteoarthritis. His age makes an apophysitis unlikely, but there may be some features of this when his knee pain initiated in his teenage years.
A NextGen LOGIQ e machine (Figure 1) with a high frequency linear array transducer (4-12MHz) used to perform the scan. A high frequency was employed in order to provide high quality superficial images. The gain and focal depth were adjusted to ensure the relevant structures were optimised in the imaging. The knee joint was examined with the patient sitting on the plinth with the knee in extension and at 30 degrees of flexion. An assessment of the knee joint was carried out which included transverse and longitudinal sections of relevant structures. Dynamic assessments were performed, testing the integrity of the structures as required.
Report:
“Right knee: There is no evidence of effusion or synovitis within the suprapatellar, prefemoral, prepatellar or infrapatellar bursa, although intra-articular pathology cannot be excluded with ultrasound.
The quadriceps tendon appears normal with normal fibrillar pattern, no evidence of tears, calcification or enthesitis.
The medial and lateral collateral ligaments appear intact. Insertion of the iliotibial band at Gerdy’s tubercle appears normal.
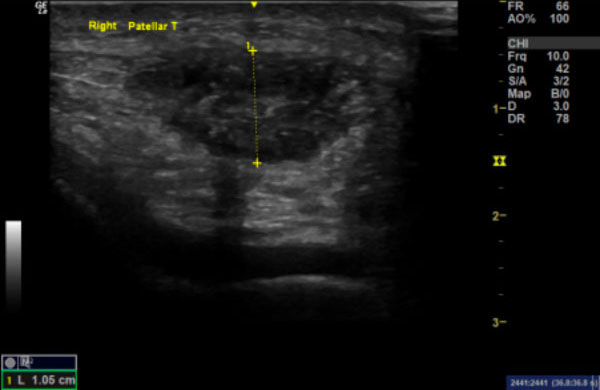
The right patella tendon appears thickened with loss of normal internal fibrillar pattern. There are numerous small calcifications throughout the tendon and patchy hypoechoic zones. There is a small amount of neovascularisation in the patella tendon on power doppler.
The right patella tendon measures 0.77cm in longitudinal section, compared to 0.56cm in the left patella tendon.
These findings are consistent with patellar tendinopathy”
Images 1a & 1b:
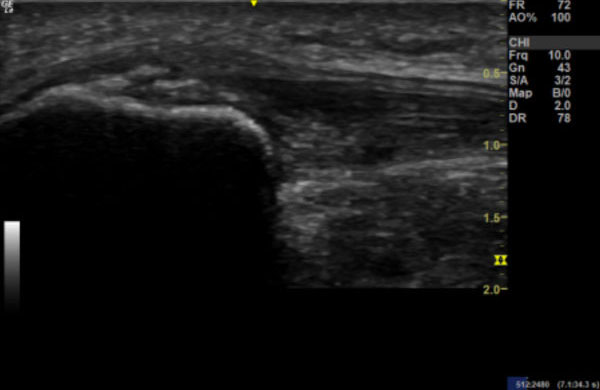
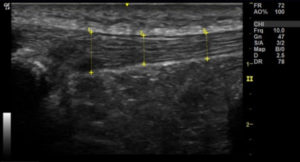
Image 1a: Longitudinal section of right patella tendon demonstrating loss of normal internal fibrillar pattern, focal hypoechoic areas and patchy calcification (arrows). Image 1b shows the right patella tendon in transverse section.
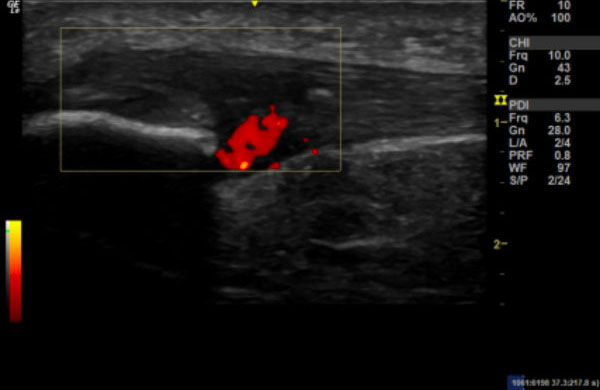
Image 2:
Longitudinal section of the right patella, with power Doppler to demonstrate neovascularisation.
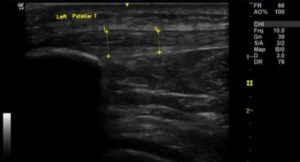
Images 3a & 3b:
Image 3a: Longitudinal section of right patella tendon showing thickening compared to Image 3b: longitudinal section of the left patella tendon.
Patella tendinopathy is traditionally a clinical diagnosis (Malliaras et al. 2015), characterised by pain in the patella tendon at the inferior pole of the patella; which increases with higher demand on the knee extensors (notably jumping/explosive activities).
Patella tendinopathy accounts for 10% of clinical knee diagnoses (Rosen et al. 2022). It commonly affects both men and women; however, in sports such as volleyball, it is reported as a higher incidence in men (up to 50%) compared to women (21%) (Keefer Hutchison et al. 2020). It is traditionally associated in sport with recurrent jumping movements, notably reported as being as prevalent as 11.8- 14.4% in recreational volleyball and basketball players (Zwerver, Bredeweg, et al. 2011); but more recently it is acknowledged to occur in any activity which causes high loading of the tendon through rapid change of direction, speed, or repetitive movements (Sprague et al. 2018). As well as having a high point prevalence, it has a high recurrence rate of 49% (Cook et al. 1997) and more than 50% of athletes may subsequently retire from sport due to the pain (Kettunen et al. 2002).
Ultrasound has many uses within medicine, particularly in imaging within musculoskeletal medicine (Jackson et al. 2021). An ultrasound machine uses sound waves to produce a two-dimensional image of a three-dimensional structure (Grogan and Mount 2022). An ultrasound machine sends an electrical signal to the probe, comprised of piezoelectrical crystals on the contact surface (Fischetti and Scott 2007). When electrical current passes through these crystals, soundwaves are produced, as they pass through the body, they reflect off different structures.
Soundwaves which then return to the probe are converted into an image (Smith and Finnoff 2009).
Tendons, which connect muscles to bone, need to withstand high tensile forces (Thorpe and Screen 2016), are comprised of a very high collagen content (Franchi et al. 2007). The collagen is grouped into fibrils which are later bound into fibres and fascicles which are all bound together in connective tissue called endotendon (Kannus 2000). Most free tendon is surrounded by paratenon, but where tendons pass through tight structures, or around bony prominence, they are surrounded by a tendon sheath (Kannus 2000). Fibres within a tendon are orientated in the direction of which tensile forces are applied (Franchi et al. 2007). This means that in the patella tendon, the fibres are aligned along the long axis of the tendon (Agergaard et al. 2021); which is visualised in Image 3b. On ultrasound, this regular alignment of tendon structure is seen as multiple parallel and closely spaced echogenic lines (McAuliffe et al. 2016; Dragoo et al. 2012). Due to the parallel nature of these lines, tendons are prone to exhibiting anisotropy. Therefore, the structures are best seen when the beam is perpendicular to the orientation of the fibres (Simard 2020; Bright et al. 2017).
This fibrillar pattern becomes disrupted when the tendon undergoes tendinopathic changes which is visualised on ultrasound as increased spacing of the hyperechoic fibrillar lines, reduced echogenicity, thickening of the tendon, and calcification can be present (Hodgson et al. 2012). Power doppler can show neovascularization (Richards et al. 2010). These changes are now described as “tendinosis” or “tendinopathy”, as they imply a degenerative pathological process as opposed to the previous term “tendinitis” which suggested inflammation. On a molecular level, risk factors are associated with tendinosis; increased age results in change to their collagen structure and water content (Franchi et al. 2007) as well as a poorer vascular supply to the tendons (Splittgerber and Ihm 2019).
Ultrasound imaging is suited to a musculoskeletal setting. The clinician is able to supplement their clinical examination with immediate access to imaging allowing for a much more accurate and prompter diagnosis to be made. Comparatively ultrasound imaging may be available on referral to a third party, but asking a dedicated sonographer to report on a region (for example a knee) will result in a list of structures being checked. This often will negate the clinical findings and report structural changes which may not be relevant to the clinical assessment. An example of this might be tendon changes in an asymptomatic knee; or in different forms of imaging, incidental disc bulges in MRI scans (Kamath et al. 2009). Utilising the imaging to detect abnormalities is clearly useful, but being able to assess for normal structures is also vital to complete an assessment. When assessing soft tissue structures, ultrasound allows for immediate dynamic assessment of these structures. An example would be a subluxing tendon, or gapping of a joint due to ligament damage. This dynamic testing is not possible with other forms of imaging, such an MRI or CT scans (Sikdar et al. 2014). Comparatively, if a finding is not clearly pathological, a benefit of ultrasound is that an immediate assessment of the contralateral limb can be made – allowing assessment of whether this is a pathological change. Ultrasound allows for the probe to be placed exactly where the pain is being felt, which can have a more targeted approach to assessing for painful structures (Pirri et al. 2021). Furthermore, pathology can immediately be assessed through the use of power or colour doppler. In many ultrasound assessments, if an area of concern is identified by the clinician, then there may be an opportunity to treat immediately, using an ultrasound guided injection (Nakashima et al. 2022). Once a diagnosis has been made, there is a supported use for regular assessments to compare progression of tissue appearance under ultrasound imaging throughout the rehabilitation (Bright et al. 2017).
Of course, there are limitations to ultrasound as an imaging modality. Firstly, there is a large variety in the experience and skill level of the machine operator. It takes a clinician who is well practiced in musculoskeletal medicine and able to use the ultrasound machine effectively to be able to generate accurate images and correlate them to their clinical findings. Without this, a lot of the benefits outlined above are negated. There are various different machines with different quality of images produced, therefore, there can be a wide variety in what structures can and cannot be accurately assessed (Nakashima et al. 2022). It is apparent that there are structures which are easily and accurately assessed with ultrasound, but some structures are more difficult, or impossible to see on ultrasound. In the knee, we are unable to see the deep ligament of the anterior cruciate ligament (ACL); this inability to scan deeper structures or see through bone are limitations (Morag and Yang 2017; Yablon et al. 2014; Lee and Chow 2007).
Magnetic resonance imaging (MRI) provides detailed images with high spatial resolution of anatomic structures and allows for good contrast between normal and abnormal tendon tissue to be differentiated (Steinbach 2022). An MRI of the knee can help provide a clear image of multiple structures at once, including the deeper structures such as the ACL. On MRI, patella tendinopathy is shown as a focal increase of signal within the tendon and also thickening of the tendon (Hodgson et al. 2012). Measurements of >7.0mm on MRI have been shown to also be an accurate clinical predictor of patella tendinopathy, with a sensitivity of 100% and a specificity of 89.4% (Nishida et al. 2021).
MRI scanning is a more expensive modality compared to ultrasound and is often a sparse resource in many areas – with either a long waiting list or complete lack of access in some settings. In a private setting this is different, but depending on what structure you are assessing, might not provide you with any more information than a point of care ultrasound (POCUS) assessment (Nakashima et al. 2022). Depending on the region of scanning, scans are long in length and require the patient to lie completely still, any movement can lead to artefact (Hodgson et al. 2012). Although they produce highly detailed images, US has better contrast and spatial resolution to detect small calcifications and foreign bodies (Warden and Brukner 2003). In tendinopathy, the tendon will thicken, with MRI there can be an association of false positive when the angle the tendon comes off the patella is too steep, due to an increase in signal intensity (Erickson et al. 1993; Erickson et al. 1991; Karantanas et al. 2001).
Outside of issues specific to musculoskeletal imaging, there are relative contraindications to MRI scanning, which do not exist with ultrasound. These include metal in the body, such as with pacemakers, intramedullary nails or metal fragments in the eyes (Streif and Hirschmann 2020). Additionally, patients who suffer from claustrophobia will struggle to tolerate MRI scanners (Hudson et al. 2022).
Computerised tomography (CT) scanning can be used to assess structures of the knee. Predominantly a CT scan can provide accurate assessment of bony changes, but has poor assessment of soft tissue structures (Kalke et al. 2012). CT scanning has an associated risk of radiation, which is infinitely more than ultrasound or MRI imaging of the area. CT scanning is not used to image patella tendons routinely.
Although ultrasound imaging is well suited to assessment of the patella tendon, it is worthwhile appreciating the other structures of the knee which can be visualised. The femoral trochlea and joint line can be visualised, although the full articular surface cannot be assessed. The quadriceps tendon and associated fat pads (suprapatellar and prefemoral) can also be seen. On the medial side of the knee, the medial collateral ligament (MCL), medial meniscus and tendon structures of the pes anserine tendons can be assessed. On the lateral side, the lateral collateral ligament (LCL), iliotibial band and its insertion into Gerdy’s tubercle can be assessed. Anteriorly, we know we can assess the patella and the patella tendon, but assessment of the patella bursae and Hoffa’s fat pad is recommended. Posteriorly, assessment of the hamstring muscles and tendons can take place, incorporating screening for Baker’s cyst and thickening of the posterior cruciate ligament (PCL).
Comparisons of the tendon with changes on imaging and direct visualisation during surgery allow for assessments of sensitivity and specificity to be made (Hodgson et al. 2012). There is limited evidence generated from surgeries of patella tendinosis given the good response to conservative management. However, in detecting lateral epicondyle tendinosis, ultrasound and MRI have both correlated equally (Potter et al. 1995). However, comparisons of ultrasound and MRI with surgical findings in the shoulder suggest ultrasound may be slightly more sensitive, but less specific than MRI for detecting tendinosis (Martín-Hervás et al. 2001). Comparatively when detecting calcific changes, ultrasound is recognised to be significantly more sensitive than MRI (Hodgson et al. 2012). A study comparing ultrasound and MRI with a clinical diagnosis of patella tendinopathy found ultrasound was more sensitive than MRI, but similar specificity. Interestingly, the specificity of ultrasound was further improved when using power doppler in this study (Warden et al. 2007). Additionally, when assessing for patella tendinopathy, ultrasound has been shown to have a sensitivity of 58% and a specificity of 94%. In comparison, MRI has a sensitivity of 78% and a specificity of 86% (Warden and Brukner 2003).
Although there are some reports of having tendinopathic changes in an asymptomatic knee; it is important to acknowledge that asymptomatic changes are associated with progression to symptomatic patella tendinopathy (Splittgerber and Ihm 2019). Therefore, detection of tendinopathic changes in an asymptomatic patient, will allow a clinician to guide their management plan.
Patella tendinopathy can be managed with strict rest; however, this shows prolonged recovery times and high recurrence rates (Malliaras et al. 2015). There is evidence which can show if left untreated, patella tendinopathy may lead to tendon tears or rupture (Rosso et al. 2015; Golman et al. 2020).
There is a convincing evidence that an eccentric loading programme has positive outcomes (Breda et al. 2021; Visnes and Bahr 2007; Lee et al. 2020). However, in some populations, such as athletes in season, programmes like this were shown to be too aggressive and resulted in a high level of irritability to the tendon (Couppé et al. 2015). Eccentric loading generates greater mechanical stimulation of the patella tendon, which in turn promotes remodelling of collagen fibres within the tendon (Andriolo et al. 2019; Quinlan et al. 2019).
A vital part of the clinical assessment is to highlight potential predisposing risk factors, such as abnormal biomechanics. Therefore, purely adhering to an impersonalised eccentric loading program fails to address underlying potential biomechanical abnormalities (Sprague et al. 2018).
Management of patella tendinopathy should begin by assessing for correctable risk factors. This might be biomechanical or intrinsic, such as arch position, limb rotation or valgus/varus deformities of the knee or issues along the kinetic chain (Sprague et al. 2018). Extrinsic factors, such as assessing footwear, discussing training surface etc. should be reviewed. Additionally, assessment of load needs to take place, be this distance covered or jumps performed; depending on the sport and precipitating factors (Muaidi 2020).
The patient’s load should be addressed. Often a period of complete unloading (rest) will need to occur. This is then increased gradually alongside a strength building program. As outlined above, eccentric loading programmes are associated with good results, especially short term (Malliaras et al. 2015). More recently, a heavy slow resistance (HSR) program has shown evidence of more favourable outcomes (Kongsgaard et al. 2009). This research showed improved tendon morphological changes and subjective function when compared to eccentric exercises alone. Breda et al. found a 4-stage approach to tendon loading led to better subjective outcomes at 24 weeks, compared to eccentric loading exercise. The stages to this approach are: isometric, isotonic, energy storage (explosive) and sport-specific exercises (Breda et al. 2021).
If a physical therapy approach does not suit the patient or does not result in significant improvement, then there are other modalities of treatment to consider. Platelet rich plasma (PRP) injections are used in numerous soft tissue injuries, particularly patella tendinopathy (Johal et al. 2019; Zayni et al. 2015). The evidence behind the benefits are varied with a recent critical analysis suggesting PRP is best utilised as an adjunct treatment alongside physical therapy (Marigi et al. 2022). Hyaluronic acid is understood to have some benefit from an analgesic perspective, particularly in acute tendinopathic changes (Fogli et al. 2017), but further research is needed to understand its long term place in management. There is limited research into prolotherapy and patella tendons, however, there does seem some benefit when these injections have been used in treatment of Osgood-Schlatter disease (Topol et al. 2011). Steroid injection to the patella tendon is rarely used anymore, due to risk of tendon rupture and a high rate of symptom relapse in the long term (Rodriguez Merchan 2013). Any of these injectable modalities could be utilised at time of diagnosis by a clinician under ultrasound guidance.
Extracorporeal shockwave therapy (ESWT) is an emerging treatment modality for tendon issues (Li et al. 2018; Huisstede et al. 2011). Initially early research suggested little change in symptoms from ESWT on patella tendinopathy (Zwerver, Hartgens, et al. 2011); however, more recent evidence does show encouraging outcomes on symptoms and reported pain (Zhang et al. 2020).
Surgical approach is consider with the recognised open surgical technique to split the tendon longitudinally, excise any abnormal looking tissue and resect a section of the inferior patella (Dan et al. 2019). Results in a small group of 38 knees showed 82% being able to return to sport and 63% of these knees were totally symptom free (Ferretti et al. 2002). Comparatively, arthroscopic approach to shave the dorsal side of the patella showed good short-term pain relief and aim to return to sport within 8 weeks, however, longer term outcomes are not as fully understood (Willberg et al. 2007). Comparatively, surgery and eccentric loading tend to have similar outcomes, but eccentric loading is less invasive and most cost efficient. It is recommended that surgery be explored as an option only if eccentric/HSR loading programmes are unsuccessful after 3-6 months (Marigi et al. 2022).
In this case, subsequent to clinical examination, ultrasound was used to confirm a diagnosis of patella tendinopathy. Although a clinical diagnosis, the beneficial role of ultrasound in this setting is highlighted by this case. It offers an immediate aid to diagnosis, allowed comparison to the contralateral limb, allowed measurements to be taken, allowed for power doppler assessment of the tendon and could have been utilised to guide an injection if appropriate – further reinforcing the possibility of a “one stop clinic”. Throughout the rehabilitation period, regular imaging of the healing tissue can guide treatment. Ultrasound has been shown to have a high sensitivity, although depending on operator experience, potentially not as high as MRI. However, specificity of ultrasound is better than MRI. This means clinically, if tendinopathy is seen on ultrasound, we can be very sure this is the pathology. It is generally accepted that a progressive eccentric loading program with heavy slow resistance has favourable outcomes. There are injectable adjuncts which appear to have some benefit to recovery, however, well designed randomised controlled studies are still needed to recommend these modalities in place of physical therapy. Surgical options exist for tendons which are not improving.
Agergaard, A.-S., Svensson, R.B., Malmgaard-Clausen, N.M., Couppé, C., Hjortshoej, M.H., Doessing, S., Kjaer, M., and Magnusson, S.P., 2021. Clinical Outcomes, Structure, and Function Improve With Both Heavy and Moderate Loads in the Treatment of Patellar Tendinopathy: A Randomized Clinical Trial. The American journal of sports medicine, 49(4), pp.982–993.
Andriolo, L., Altamura, S.A., Reale, D., Candrian, C., Zaffagnini, S., and Filardo, G., 2019. Nonsurgical Treatments of Patellar Tendinopathy: Multiple Injections of Platelet-Rich Plasma Are a Suitable Option: A Systematic Review and Meta-analysis. The American journal of sports medicine, 47(4), pp.1001–1018.
Breda, S.J., Oei, E.H.G., Zwerver, J., Visser, E., Waarsing, E., Krestin, G.P., and de Vos, R.-J., 2021. Effectiveness of progressive tendon-loading exercise therapy in patients with patellar tendinopathy: a randomised clinical trial. British journal of sports medicine, 55(9), pp.501–509.
Bright, J.M., Fields, K.B., and Draper, R., 2017. Ultrasound Diagnosis of Calf Injuries. Sports health, 9(4), pp.352–355.
Cook, J.L., Khan, K.M., Harcourt, P.R., Grant, M., Young, D.A., and Bonar, S.F., 1997. A cross sectional study of 100 athletes with jumper’s knee managed conservatively and surgically. The Victorian Institute of Sport Tendon Study Group. British journal of sports medicine, 31(4), pp.332–6.
Couppé, C., Svensson, R.B., Silbernagel, K.G., Langberg, H., and Magnusson, S.P., 2015. Eccentric or Concentric Exercises for the Treatment of Tendinopathies? The Journal of orthopaedic and sports physical therapy, 45(11), pp.853–63.
Dan, M., Phillips, A., Johnston, R. V, and Harris, I.A., 2019. Surgery for patellar tendinopathy (jumper’s knee). The Cochrane database of systematic reviews, 9(9), p.CD013034.
Dragoo, J.L., Johnson, C., and McConnell, J., 2012. Evaluation and treatment of disorders of the infrapatellar fat pad. Sports medicine (Auckland, N.Z.), 42(1), pp.51–67.
Erickson, S.J., Cox, I.H., Hyde, J.S., Carrera, G.F., Strandt, J.A., and Estkowski, L.D., 1991. Effect of tendon orientation on MR imaging signal intensity: a manifestation of the magic angle phenomenon.. Radiology, 181(2), pp.389–92.
Erickson, S.J., Prost, R.W., and Timins, M.E., 1993. The magic angle effect: background physics and clinical relevance. Radiology, 188(1), pp.23–5.
Ferretti, A., Conteduca, F., Camerucci, E., and Morelli, F., 2002. Patellar Tendinosis. The Journal of Bone and Joint Surgery-American Volume, 84(12), pp.2179–2185.
Fischetti, A.J. and Scott, R.C., 2007. Basic ultrasound beam formation and instrumentation. Clinical techniques in small animal practice, 22(3), pp.90–2.
Fogli, M., Giordan, N., and Mazzoni, G., 2017. Efficacy and safety of hyaluronic acid (500-730kDa) Ultrasound guided injections on painful tendinopathies: a prospective, open label, clinical study. Muscles, ligaments and tendons journal, 7(2), pp.388–395.
Franchi, M., Trirè, A., Quaranta, M., Orsini, E., and Ottani, V., 2007. Collagen structure of tendon relates to function. TheScientificWorldJournal, 7, pp.404–20.
Golman, M., Wright, M.L., Wong, T.T., Lynch, T.S., Ahmad, C.S., Thomopoulos, S., and Popkin, C.A., 2020. Rethinking Patellar Tendinopathy and Partial Patellar Tendon Tears: A Novel Classification System. The American journal of sports medicine, 48(2), pp.359–369.
Grogan, S.P. and Mount, C.A., 2022. Ultrasound Physics and Instrumentation. StatPearls Publishing. Hodgson, R.J., O’Connor, P.J., and Grainger, A.J., 2012. Tendon and ligament imaging. The British Journal of Radiology, 85(1016), p.1157.
Hudson, D.M., Heales, C., and Meertens, R., 2022. Review of claustrophobia incidence in MRI: A service evaluation of current rates across a multi-centre service. Radiography (London, England : 1995), 28(3), pp.780–787.
Huisstede, B.M.A., Gebremariam, L., van der Sande, R., Hay, E.M., and Koes, B.W., 2011. Evidence for effectiveness of Extracorporal Shock-Wave Therapy (ESWT) to treat calcific and non-calcific rotator cuff tendinosis--a systematic review. Manual therapy, 16(5), pp.419–33.
Jackson, S.S., Le, H.M., Kerkhof, D.L., and Corrado, G.D., 2021. Point-of-Care Ultrasound, the New Musculoskeletal Physical Examination. Current sports medicine reports, 20(2), pp.109–112. Johal, H., Khan, M., Yung, S.-H.P., Dhillon, M.S., Fu, F.H., Bedi, A., and Bhandari, M., 2019. Impact of Platelet Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis. Sports health, 11(4), pp.355–366.
Kalke, R.J., Di Primio, G.A., and Schweitzer, M.E., 2012. MR and CT arthrography of the knee. Seminars in musculoskeletal radiology, 16(1), pp.57–68.
Kamath, S., Jain, N., Goyal, N., Mansour, R., and Mukherjee, K., 2009. Incidental findings on MRI of the spine. Clinical radiology, 64(4), pp.353–61.
Kannus, P., 2000. Structure of the tendon connective tissue. Scandinavian journal of medicine & science in sports, 10(6), pp.312–20.
Karantanas, A.H., Zibis, A.H., and Papanikolaou, N., 2001. Increased signal intensity on fat-suppressed three dimensional T1-weighted pulse sequences in patellar tendon: magic angle effect? Skeletal radiology, 30(2), pp.67–71.
Keefer Hutchison, M., Patterson, C., Cuddeford, T., Dudley, R., Sorenson, E., and Brumitt, J., 2020. Low prevalence of patellar tendon abnormality and low incidence of patellar tendinopathy in female collegiate volleyball players. Research in sports medicine (Print), 28(2), pp.155–167.
Kettunen, J.A., Kvist, M., Alanen, E., and Kujala, U.M., 2002. Long-term prognosis for jumper’s knee in male athletes. A prospective follow-up study. The American journal of sports medicine, 30(5), pp.689–92. Kongsgaard, M., Kovanen, V., Aagaard, P., Doessing, S., Hansen, P., Laursen, A.H., Kaldau, N.C., Kjaer, M., and Magnusson, S.P., 2009. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scandinavian journal of medicine & science in sports, 19(6), pp.790–802.
Lee, M.J. and Chow, K., 2007. Ultrasound of the knee. Seminars in musculoskeletal radiology, 11(2), pp.137–48. Lee, W.-C., Ng, G.Y.-F., Zhang, Z.-J., Malliaras, P., Masci, L., and Fu, S.-N., 2020. Changes on Tendon Stiffness and Clinical Outcomes in Athletes Are Associated With Patellar Tendinopathy After Eccentric Exercise. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine, 30(1), pp.25–32.
Li, X., Zhang, L., Gu, S., Sun, J., Qin, Z., Yue, J., Zhong, Y., Ding, N., and Gao, R., 2018. Comparative effectiveness of extracorporeal shock wave, ultrasound, low-level laser therapy, noninvasive interactive neurostimulation, and pulsed radiofrequency treatment for treating plantar fasciitis: A systematic review and network meta-analysis. Medicine, 97(43), p.e12819.
Malliaras, P., Cook, J., Purdam, C., and Rio, E., 2015. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. The Journal of orthopaedic and sports physical therapy, 45(11), pp.887–98.
Marigi, E.M., Buckley, P., Razi, F., Abbas, M.J., Jildeh, T.R., Camp, C.L., Krych, A.J., and Okoroha, K.R., 2022. Patellar Tendinopathy: Critical Analysis Review of Current Nonoperative Treatments. JBJS Reviews, 10(3). Martín-Hervás, C., Romero, J., Navas-Acién, A., Reboiras, J.J., and Munuera, L., 2001. Ultrasonographic and magnetic resonance images of rotator cuff lesions compared with arthroscopy or open surgery findings. Journal of shoulder and elbow surgery, 10(5), pp.410–5.
McAuliffe, S., McCreesh, K., Culloty, F., Purtill, H., and O’Sullivan, K., 2016. Can ultrasound imaging predict the development of Achilles and patellar tendinopathy? A systematic review and meta-analysis. British journal of sports medicine, 50(24), pp.1516–1523.
Morag, Y. and Yang, L.J.S., 2017. Imaging Nerve Pathology of the Knee: Magnetic Resonance Imaging and Ultrasound. Seminars in musculoskeletal radiology, 21(2), pp.122–136.
Muaidi, Q.I., 2020. Rehabilitation of patellar tendinopathy. Journal of musculoskeletal & neuronal interactions, 20(4), pp.535–540.
Nakashima, Y., Sunagawa, T., Shinomiya, R., Kodama, A., and Adachi, N., 2022. Point-of-care ultrasound in musculoskeletal field. Journal of medical ultrasonics (2001), 49(4), pp.663–673.
Nishida, Y., Nishino, T., Tanaka, K., Onishi, S., Kanamori, A., and Yamazaki, M., 2021. An Objective Measure of Patellar Tendon Thickness Based on Ultrasonography and MRI in University Athletes. Journal of clinical medicine, 10(18).
Pirri, C., Ricci, V., Stecco, C., and Özçakar, L., 2021. Clinical and Ultrasound Examination of the Thoracolumbar Fascia: The Hands and the Probe Together. American Journal of Physical Medicine & Rehabilitation, 100(10), pp.e157–e158.
Potter, H.G., Hannafin, J.A., Morwessel, R.M., DiCarlo, E.F., O’Brien, S.J., and Altchek, D.W., 1995. Lateral epicondylitis: correlation of MR imaging, surgical, and histopathologic findings. Radiology, 196(1), pp.43–6. Quinlan, J.I., Narici, M. V, Reeves, N.D., and Franchi, M. V, 2019. Tendon Adaptations to Eccentric Exercise and the Implications for Older Adults. Journal of functional morphology and kinesiology, 4(3). Richards, P.J., McCall, I.W., Day, C., Belcher, J., and Maffulli, N., 2010. Longitudinal microvascularity in Achilles tendinopathy (power Doppler ultrasound, magnetic resonance imaging time-intensity curves and the Victorian Institute of Sport Assessment-Achilles questionnaire): a pilot study. Skeletal radiology, 39(6), pp.509–21.
Rodriguez-Merchan, E.C., 2013. The treatment of patellar tendinopathy. Journal of orthopaedics and traumatology : official journal of the Italian Society of Orthopaedics and Traumatology, 14(2), pp.77–81. Rosen, A.B., Wellsandt, E., Nicola, M., and Tao, M.A., 2022. Clinical Management of Patellar Tendinopathy. Journal of athletic training, 57(7), pp.621–631.
Rosso, F., Bonasia, D.E., Cottino, U., Dettoni, F., Bruzzone, M., and Rossi, R., 2015. Patellar tendon: From tendinopathy to rupture. Asia-Pacific journal of sports medicine, arthroscopy, rehabilitation and technology, 2(4), pp.99–107.
Sikdar, S., Wei, Q., and Cortes, N., 2014. Dynamic ultrasound imaging applications to quantify musculoskeletal function. Exercise and sport sciences reviews, 42(3), pp.126–35.
Simard, R., 2020. Ultrasound Imaging of Orthopedic Injuries. Emergency medicine clinics of North America, 38(1), pp.243–265.
Smith, J. and Finnoff, J.T., 2009. Diagnostic and interventional musculoskeletal ultrasound: part 1. Fundamentals. PM & R : the journal of injury, function, and rehabilitation, 1(1), pp.64–75. Splittgerber, L.E. and Ihm, J.M., 2019. Significance of Asymptomatic Tendon Pathology in Athletes. Current sports medicine reports, 18(6), pp.192–200.
Sprague, A.L., Smith, A.H., Knox, P., Pohlig, R.T., and Grävare Silbernagel, K., 2018. Modifiable risk factors for patellar tendinopathy in athletes: a systematic review and meta-analysis. British journal of sports medicine, 52(24), pp.1575–1585.
Steinbach, L.S., 2022. MR Imaging of the Knee. Magnetic resonance imaging clinics of North America, 30(2), p.xi.
Streif, M. and Hirschmann, A., 2020. Magnetic resonance arthrography. Der Radiologe, 60(3), pp.273–284. Thorpe, C.T. and Screen, H.R.C., 2016. Tendon Structure and Composition. Advances in experimental medicine and biology, 920, pp.3–10.
Topol, G.A., Podesta, L.A., Reeves, K.D., Raya, M.F., Fullerton, B.D., and Yeh, H., 2011. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics, 128(5), pp.e1121-8.
Visnes, H. and Bahr, R., 2007. The evolution of eccentric training as treatment for patellar tendinopathy (jumper’s knee): a critical review of exercise programmes. British journal of sports medicine, 41(4), pp.217–23. Warden, S.J. and Brukner, P., 2003. Patellar tendinopathy. Clinics in sports medicine, 22(4), pp.743–59. Warden, S.J., Kiss, Z.S., Malara, F.A., Ooi, A.B.T., Cook, J.L., and Crossley, K.M., 2007. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. The American journal of sports medicine, 35(3), pp.427–36.
Willberg, L., Sunding, K., Ohberg, L., Forssblad, M., and Alfredson, H., 2007. Treatment of Jumper’s knee: promising short-term results in a pilot study using a new arthroscopic approach based on imaging findings. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA, 15(5), pp.676–81.
Yablon, C.M., Melville, D.M., and Jacobson, J.A., 2014. Ultrasound of the knee. AJR. American journal of roentgenology, 202(3), p.W284.
Zayni, R., Thaunat, M., Fayard, J.-M., Hager, J.-P., Carrillon, Y., Clechet, J., Gadea, F., Archbold, P., and Sonnery Cottet, B., 2015. Platelet-rich plasma as a treatment for chronic patellar tendinopathy: comparison of a single versus two consecutive injections. Muscles, ligaments and tendons journal, 5(2), pp.92–8.
Zhang, Z.J., Lee, W.C., and Fu, S.N., 2020. One Session of Extracorporeal Shockwave Therapy-Induced Modulation on Tendon Shear Modulus is Associated with Reduction in Pain. Journal of sports science & medicine, 19(2), pp.309–316.
Zwerver, J., Bredeweg, S.W., and van den Akker-Scheek, I., 2011. Prevalence of Jumper’s knee among nonelite athletes from different sports: a cross-sectional survey. The American journal of sports medicine, 39(9), pp.1984–8.
Zwerver, J., Hartgens, F., Verhagen, E., van der Worp, H., van den Akker-Scheek, I., and Diercks, R.L., 2011. No effect of extracorporeal shockwave therapy on patellar tendinopathy in jumping athletes during the competitive season: a randomized clinical trial. The American journal of sports medicine, 39(6), pp.1191–9.