
A 49 year old woman presented to MSK clinic with 8 months history of right (R) forefoot pain of non-traumatic onset. The pain gets aggravated by weight bearing type of activities and she feels as if she “was walking on a pebble”. Symptoms resolve at rest. She can occasionally feel sharp “electric like “sensation radiating to her 3rd and 4th toes. She reported no pain at night, denied lower back pain or signs of “sciatica”. Medical history is inclusive of hypertension, hyperlipidaemia and elevated BMI with weight at 15 stones, but the patient is otherwise fit and well. She takes non-steroidal anti-inflammatories (NSAIDs) intermittently to reduce the pain with limited benefit. She works in an office environment and admitted to not being very active.
Clinical examination revealed normal, unaided gait, no forefoot swelling, no lower limb or foot muscles atrophy, no toes deformities, no signs of flat or high arched foot and no visible calluses on the plantar aspect of the forefoot. The patient had full R ankle range of movement (ROM) and no gastro-soleus complex tightness on Silfverskiöld test. There was pain on thumb-index finger squeeze test over 3rd intermetatarsal space and on metatarsal heads transverse compression. There was no pain on active or passive 1st,2nd, 3rd, 4th or 5th metatarsal – phalangeal joint ( MTPJ ) dorsal flexion ( DF ) or plantar flexion ( PF ) with painless direct palpation over all metatarsal heads ( MH ) and shafts.
The likely diagnosis of R forefoot pain was 3rd interspace Morton’s neuroma , however, examination alone, could not unquestionably exclude other potential pathologies to include: 3rd or 4th MTPJ capsulitis/synovitis, plantar plate pathology, metatarsal stress fracture, Freiberg’s infraction, MTPJ osteoarthritis ( OA ) or flexor digitorum tendinopathy .
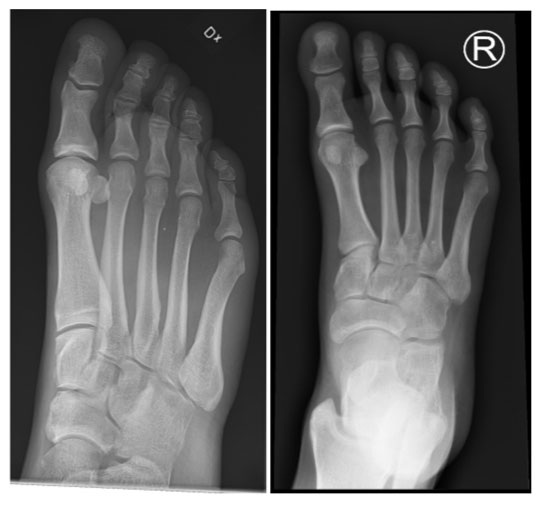
The patient had R forefoot x-ray (AP and oblique views) without any pathology identified, which helped further in narrowing probability of correct diagnosis.
X-ray report: “no abnormality seen“
Figure 1: Normal foot xray AP and oblique
A Phillips Epiq Elite machine (Figure 1) with a high frequency linear array transducer (5-18MHz) was used to perform high quality image and to increase diagnostic accuracy. The foot was examined with patient laying in supine position on the plinth. The ankle was positioned in neutral with the foot free to allow manipulation by the examiner during scanning, giving easy access to plantar and dorsal aspects of the forefoot, and allowing for dynamic sonographic manoeuvres. An assessment of the forefoot was carried out, which included transverse and longitudinal sections of the area of interest.
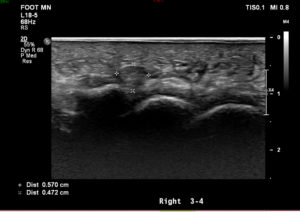
US findings and reports: “There is a tender 8 mm hypoechoic partially compressible mass in the 3rd/4th intermetatarsal space suggestive of a neuroma bursal complex. The neuroma component measures about 4 mm. No mass in the 2nd/3rd intermetatarsal space is identified. No associated tenderness. No plantar plate disruption / tear or active inflammation noted in the MTPJs. No other cause for forefoot pain seen”.

These clinical and ultrasound (US) findings support diagnosis of Morton’s neuroma. Cohen SL, 2016, in his study compared the sonographic findings of the interdigital mass to the pathological findings after surgical excision of the mass. They concluded, that the interdigital masses were a combined appearance of the degenerated nerve, perineural scarring, tangled vessels, and scarred inter-metatarsal bursa and suggested a term bursal – neuromal complex.
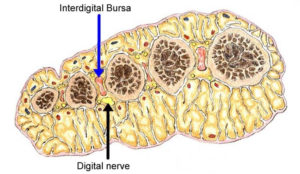
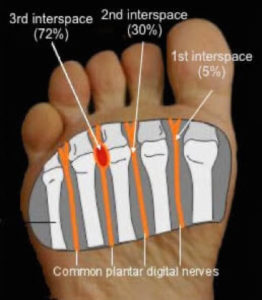
Morton neuroma (MN) (peculiar painful affection) was first described in 1876 (Morton TG, 1876).The most frequent location is 3rd web space ( 72% ) , followed by 2nd web space ( 30%) and quite rare in 4th web space ( 5%), (Giakoumis M, 2013). It is characterised by neural degeneration with epineural and endoneurial vascular hyalinisation and perineural fibrosis around a plantar digital nerve (Zanetti M, 1999). The exact cause of an interdigitial neuroma is unclear. It has been hypothesized that the common plantar digital nerve is repeatedly compressed between the bottom of the foot and the edge of the transverse inter-metatarsal ligament. This results in a chronic irritation of the nerve and subsequent development of a benign fibrotic degeneration within the nerve. The most typical symptoms of Morton’s neuroma are often described as sharp and burning pain associated with dysesthesia, cramping and numbness in the toes adjacent to the neuroma, accompanied by pain. Pain typically is intermittent, as episodes often last for minutes to hours at a time and has long intervals. The contributing factors may include: tight fitting footwear, toes deformities (claw toes, bunions, hallux valgus), hypermobile joints, flat or high arched foot.
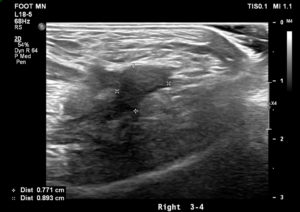
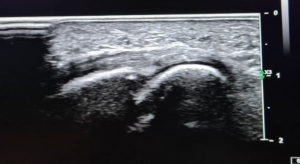
The appearance of Morton’s neuromas sonographically has been characterized as a well-defined hypoechoic mass at the level of the metatarsal heads, most commonly in the 3rd metatarsal space. The typical histological appearance of Morton’s neuromas is fusiform enlargement of the nerve resulting in an ovoid shape with the greatest dimension being length. From the patient images (Figure 4) the main findings were a hypoechoic structure of 0.771 x 0.893 cm dimension in the 3rd metatarsal space, which was partially compressible on dynamic US assessment (Figure5). Although not appreciated on attached evidence, sonographic palpation elicited pain in the area.
Quinn et al. (2000) considered clinical significance of 3D dimension of Morton’s neuroma. He found no statistical significance with regard to measurements of height and width, but found a significant difference in length between neuromas and nonneuromas. The average length of the nonneuromas (23 ± 10 mm) was much greater than that of the measured neuromas (13 ± 6 mm). No neuroma was greater than 20 mm in length. The finding of an interdigital mass longer than 20 mm should alert suspicion of an abnormality other than a neuroma and prompt different imaging modalities or biopsy for further tissue characterisation.
Figure 4, shows hypoechoic mass in longitudinal view positioned in the 3rd metatarsal space measuring 0.893 cm in length.
US has been championed as an efficient modality in detecting Morton's neuroma and equal to MRI. In a study by Bianca B et al. 2015, diagnostic sensitivity for both MRI and US was calculated at 95%. Other studies demonstrated that US is more accurate than MRI for diagnosis of MN. Analysis of pooled sensitivity showed similarity between US (90%) and MRI (93%), both being relatively high, yet, MRI (68%) showed a relatively lower specificity than US (88%) in the detection of MN ( Xu Z, 2015).
Nevertheless, there are additional diagnostic advantages of MRI in detecting other causes of forefoot pain, including abnormalities of the bone (oedema, fracture, erosion and cyst) and soft tissues ( muscle oedema or atrophy ),which some of them could be invisible to US.
Quinn et al. (2000) highlighted sonographic challenge in identifying small peripheral nerves. He showed that normal plantar digital nerve at the level of the metatarsal heads measures 1–2 mm in diameter, which is not easily visualised on US. Although most symptomatic neuromas are larger than 5 mm in diameter, size and symptoms are not absolutely related, and some symptomatic lesions may be too small to be revealed on sonography (Shapiro PP, 1995).
Symeonidis at el. 2012 suggested a high rate of false positive, incidental finding of an asymptomatic enlargement of interdigital nerve dimensions, which can lead to a false diagnosis of a Morton's neuroma. The prevalence of asymptomatic MN has been reported as 33%-54% when using ultrasound (US) or magnetic resonance imaging (MRI) for diagnosis. Sonographic evidence of Morton's neuroma is unreliable unless it is correlated with a clinical examination and reported symptoms. Clinical examination should be the gold standard for the diagnosis of a Morton's neuroma. The thumb index finger squeeze test has been reported to be the most sensitive screening method for clinical diagnosis of MN, having accuracy of around 96% (Mahadevan D, 2015).
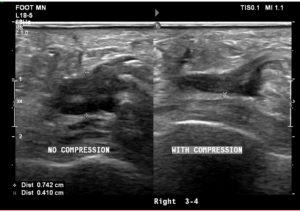
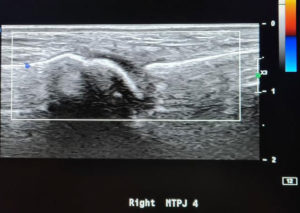
Martin T at el. 2012, demonstrated that symptomatic sonographic Mulder sign allowed for better visualisation adding to diagnostic accuracy and confidence. It is theorised that the Mulder click will only happen when there is a MN because a thickened bursa is much more pliable than a neuroma (Simmons D.N, 2008). In addition, knowing the possible pain sources to investigate when a patient has a specific site of pain will enhance the diagnostic quality of US, and will help clinician to perform US efficiently and effectively. Figure 5, illustrated partial compression of the hypoechoic mass under sonographic palpation combined with pain sensation on overpressure of the area.
A range of different pathological conditions produces forefoot pain and various or combination of imaging modalities may be required to scrutinise the aetiology of unclear forefoot pain or when more than one web space is affected.
US is used as an imaging modality of choice to facilitate diagnosis of forefoot pain due to stress fracture, typically seen in soldiers, runners, around the 2nd and 3rd metatarsals and the lower tibia. Evidence of stress fracture on US commonly appears as cortical disruption and periosteal oedema seen as hypoechoic swelling. Plain radiographs may be normal for several weeks before a callus or a fracture line appears in the case of stress fractures. Low cost, non‐invasive, easy access, ultrasound is the preferred method and may in the future usefully replace MRI in the early diagnosis of stress fracture of superficial bones (Bannal 2006).
Champagne et al.2019, compared effectiveness of US with radiography, CT scan or MRI. Their study showed that sensitivity and specificity of US was 0.93 and 0.92 for upper limb fractures , and 0.83 and 0.93 for lower limb fractures.
MTPJ instability syndrome may contribute to symptoms of forefoot pain and can masquerade symptoms of Morton’s neuroma. It can present in acute, subacute or chronic forms. Plantar plate injury is the main underlying cause of MTP joint instabilities due to its central location and multiple important attachments. Plantar plate injuries that commonly happen around 2nd or 3rd MTPJ should be distinguished from Morton’s neuroma, because a misdiagnosis would worsen the toe deformity and dysfunction. Forefoot pain due to capsular hypertrophy and MTPJ instability is sonographically best appreciated on short axis view and appears commonly as very circumferential structure of mixed echogenicity. One must be very vigilant and to avoid misdiagnosis of Morton’s neuroma, which may lead to wrong management pathway, including surgery and potential medico-legal consequences. Strong correlation between clinical examination and imaging should be the gold standard for the diagnosis to avoid such pitfalls.
Although MRI is accepted as the imaging modality of choice, US also shows a good correlation with the intraoperative finding of plantar plate injury (Klein EE, 2013). A dynamic assessment with dorsiflexion of the toe can be performed to increase the sensitivity and accuracy of US for diagnosing plantar plate injuries (McCarthy CL, 2021).
MTPJ synovitis should be considered as another differential diagnosis when considering forefoot pain. US includes an additional and very useful clinical future when assessing for small joints synovitis. On US, normal joints have smooth cortical surfaces, thin synovial recesses at the dorsal and plantar aspects , and no vascularity on Doppler US ( Umans et al 2014 ). When there is an increased amount of synovial-fluid complexes or synovial hypertrophy with intra-articular Doppler flow, active synovitis can be diagnosed on US (Gregg et al, 2007).
From the patient images (Figure 8 ) the main findings were no vascularity on Dopler US of the 3rd and 4th MTPJ.
Management for MN is usually conservative initially before escalating to infiltrative treatments and surgery if conservative therapy fails.
Conservative therapy includes: shoe modification, use of orthotics designed to limit the digital nerve compression and administration of NSAIDs. Shoes should be of appropriate size, comfortable, broad, should have a flat heel and a sufficiently thick external sole, which should not be excessively flexible. In a study by Kilmartin at el. 1994, twenty-three adult patients with Morton's neuroma of single foot were randomized to receive in-shoe adjustments, made from a hard, compressed, felt material that would either pronate or supinate both feet. The response of the neuroma pain was measured using subjective visual analogue scales, an objective examination, and the MACTAR patient-specific measure of maximal function. They reported no statistically significant reduction in pain with either type of wedge. Reviewed research also highlighted lack of high-quality evidence to inform, which conservative intervention should be the gold standard
Infiltrative therapy includes guided or blind injections of local anaesthetics or steroids.
Saygi B, et al. 2005, studied management of Morton’s neuroma as a combination of conservative and infiltrative therapy. They included footwear modification of the forefoot loading area with and without steroid injections. Outcomes were evaluated at 1 month, 6 months, and 12 months. They demonstrated greater pain improvement (at 6 months follow-up) experienced by the steroid-treated group, but this difference disappeared at 1 year of follow-up. At 12-month follow up, 82% of those treated with steroid injections had complete or partial relief of pain compared to 63% of those treated with footwear modifications alone. It is worth noticing, however, that no adverse events were reported with footwear and padding. Unfavourable events following steroid injection included dorsal skin hypopigmentation, skin atrophy and plantar fat pad atrophy.
Studies of US-guided injections have shown that mixed anaesthetics with corticosteroids infiltration produce an effect (at 3 months follow-up) that is superior to anaesthetic injections alone (Thompson CE , 2013).Other studies concentrated on the outcomes of injection accuracy for management of MN. The study by Mahadevan D, at el.2016, compared the blind technique, based on anatomical landmarks, to the US-guided technique. In that study, no significant differences were found between the injection methods, but the sample size (only 36 cases) potentially accounted for a substantial limitation that might have prevented the study from achieving a statistically significant difference.
Other researchers studied a potential prerequisite of MN size as a qualification for successful management for steroid injection. Park YH, 2018, found a cut-off value of 6.3 mm, above which larger MN failed to statistically respond to corticosteroid injections. It is worth mentioning, that most of the reported data in the literature do not support any influence of neuroma size on the outcomes ( Mahadevan D,2016).
Surgical treatment is promoted after failure of conservative or infiltrative therapies. Some studies, however, highlighted that the patient-reported outcomes after resection of a symptomatic Morton's neuroma are acceptable but may not be as good as earlier studies suggested (Bucknall V, 2016).
Vilisena S et al, 2018 conducted database search of case series and randomized controlled trials (RCTs) assessing patient’s satisfaction or pain improvement at an average follow-up of at least 6 months after treatment of primary MN. The evaluated evidence showed better results with invasive treatment. Although this review suggested that operative treatments lead to better results, this is mainly due to the absence of good research addressing conservative management.
A 49 year old female reported chronic,non traumatic R forefoot pain , localised to the 3rd metatarsal space, aggravated by weight bearing type activities. Clinical examination findings included local tenderness on index finger – thumb squeeze test of the 3rd metatarsal space in absence of pain and laxity on active and passive neighbouring MTPJ movements and direct palpation of the joints.
Sonographic evidence (see figure 3) showed 8 mm hypoechoic partially compressible mass in the 3rd/4th intermetatarsal space suggestive of a neuroma bursal complex, which supported further clinical suspicion of MN.
US in this case, was a very practical, diagnostic method, readily applied to identify the cause of metatarsalgia. The superficial location of structures in the foot, dynamic capability of US, and the ability to perform real-time evaluations of the pain site were also strong advantages ( see figure 5), which helped to correlate clinical findings and amplify the strength of initial clinical impression. Sonography as a real time visual modality, enhances accuracy of targeted infiltrative therapy, however, some authors would argue this does not necessary translates into additional clinical benefits to patients.
US is considered to be very effective and equal to MRI, investigation modality for detection of MN as suggested by numerous medical publications reviewed in this paper, however, its clinical success heavily depends on operator knowledge of anatomy, probe skills and experience. US as opposed to MRI, hard copy images, make it difficult to read and interpret remotely due to operator dependent US specific artefacts.
In addition supremacy of sonography, as a whole, includes no known contraindications, is typically well tolerated by patients (relatively short duration compering to MRI, open space- no risk of claustrophobia), readily available and cost effective (Bianca B, 2015).
Although the clinical diagnosis of MN has a high accuracy, controversies still exist in the most appropriate management of MN. The coexistence of MN with many other causes of forefoot pain increases the importance of correct choice of imaging modality to enhance outcomes of clinical examination in achieving an accurate diagnosis, especially, when initial conservative measures fail to obtain any satisfactory clinical improvement.
O'Connor, F.G., Wilder, R.P. and Nirschl, R., 2001. Foot Injuries in the Runner. Textbook of Running Medicine, pp.258-260.
Cohen, S.L., Miller, T.T., Ellis, S.J., Roberts, M.M. and DiCarlo, E.F., 2016. Sonography of Morton neuromas: what are we really looking at?. Journal of Ultrasound in Medicine, 35(10), pp.2191-2195.
Morton, T.G., 1876. A peculiar and painful affection of the fourth metatarsophalangeal articulation. Am J Med Sci, 71(35), p.9.
Giakoumis, M., Ryan, J.D. and Jani, J., 2013. Histologic evaluation of intermetatarsal Morton’s neuroma. Journal of the American Podiatric Medical Association, 103(3), pp.218-222.
Zanetti, M., Strehle, J.K., Kundert, H.P., Zollinger, H. and Hodler, J., 1999. Morton neuroma: effect of MR imaging findings on diagnostic thinking and therapeutic decisions. Radiology, 213(2), pp.583-588.
Bignotti, B., Signori, A., Sormani, M.P., Molfetta, L., Martinoli, C. and Tagliafico, A., 2015. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. European radiology, 25(8), pp.2254-2262.
Torriani, M. and Kattapuram, S.V., 2003. Dynamic sonography of the forefoot: the sonographic Mulder sign. American journal of roentgenology (1976), 180(4), pp.1121-1123.
Quinn, T.J., Jacobson, J.A., Craig, J.G. and van Holsbeeck, M.T., 2000. Sonography of Morton's neuromas. American Journal of Roentgenology, 174(6), pp.1723-1728.
Shapiro, P.P. and Shapiro, S.L., 1995. Sonographic evaluation of interdigital neuromas. Foot & Ankle International, 16(10), pp.604-606.
Symeonidis, P.D., Iselin, L.D., Simmons, N., Fowler, S., Dracopoulos, G. and Stavrou, P., 2012. Prevalence of interdigital nerve enlargements in an asymptomatic population. Foot & Ankle International, 33(7), pp.543-547.
Bencardino, J., Rosenberg, Z.S., Beltran, J., Liu, X. and Marty-Delfaut, E., 2000. Morton’s Neuroma: Is It Always.
Mahadevan, D., Venkatesan, M., Bhatt, R. and Bhatia, M., 2015. Diagnostic accuracy of clinical tests for Morton's neuroma compared with ultrasonography. The Journal of Foot and Ankle Surgery, 54(4), pp.549-553.
Xu, Z., Duan, X., Yu, X., Wang, H., Dong, X. and Xiang, Z., 2015. The accuracy of ultrasonography and magnetic resonance imaging for the diagnosis of Morton's neuroma: a systematic review. Clinical radiology, 70(4), pp.351-358.
Simmons, D.N., 2008. Imaging of the painful forefoot. Techniques in Foot & Ankle Surgery, 7(4), pp.238-249.
Thomson, C.E., Beggs, I., Martin, D.J., McMillan, D., Edwards, R.T., Russell, D., Yeo, S.T., Russell, I.T. and Gibson, J.A., 2013. Methylprednisolone injections for the treatment of Morton neuroma: a patient-blinded randomized trial. JBJS, 95(9), pp.790-798.
Mahadevan, D., Attwal, M., Bhatt, R. and Bhatia, M., 2016. Corticosteroid injection for Morton’s neuroma with or without ultrasound guidance: a randomised controlled trial. The Bone & Joint Journal, 98(4), pp.498-503.
Saygi, B., Yildirim, Y., Saygi, E.K., Kara, H. and Esemenli, T., 2005. Morton neuroma: comparative results of two conservative methods. Foot & ankle international, 26(7), pp.556-559.
Park, Y.H., Lee, J.W., Choi, G.W. and Kim, H.J., 2018. Risk factors and the associated cutoff values for failure of corticosteroid injection in treatment of Morton’s neuroma. International Orthopaedics, 42(2), pp.323-329.
Kilmartin, T.E. and Wallace, W.A., 1994. Effect of pronation and supination orthosis on Morton's neuroma and lower extremity function. Foot & ankle international, 15(5), pp.256-262.
Hughes, R.J., Ali, K., Jones, H., Kendall, S. and Connell, D.A., 2007. Treatment of Morton's neuroma with alcohol injection under sonographic guidance: follow-up of 101 cases. American Journal of Roentgenology, 188(6), pp.1535-1539.
Bucknall, V., Rutherford, D., MacDonald, D., Shalaby, H., McKinley, J. and Breusch, S.J., 2016. Outcomes following excision of Morton’s interdigital neuroma: a prospective study. The Bone & Joint Journal, 98(10), pp.1376-1381.
Valisena, S., Petri, G.J. and Ferrero, A., 2018. Treatment of Morton’s neuroma: a systematic review. Foot and Ankle Surgery, 24(4), pp.271-281.
Banal, F., Etchepare, F., Rouhier, B., Rosenberg, C., Foltz, V., Rozenberg, S., Koeger, A.C., Fautrel, B. and Bourgeois, P., 2006. Ultrasound ability in early diagnosis of stress fracture of metatarsal bone. Annals of the rheumatic diseases, 65(7), pp.977-978.
Champagne, N., Eadie, L., Regan, L.,Wilson, P., 2019. The effectiveness of ultrasound in the detection of fractures in adults with suspected upper or lower limb injury: a systematic review and subgroup meta-analysis. BMC Emergency Medicine, 14(3), pp.19-17.
Umans, H., Srinivasan, R., Elsinger, E. and Wilde, G.E., 2014. MRI of lesser metatarsophalangeal joint plantar plate tears and associated adjacent interspace lesions. Skeletal radiology, 43(10), pp.1361-1368.
Gregg, J. and Marks, P., 2007. Metatarsalgia: an ultrasound perspective. Australasian radiology, 51(6), pp.493-499.
Klein, E.E., Weil Jr, L., Weil Sr, L.S. and Knight, J., 2013. Musculoskeletal ultrasound for preoperative imaging of the plantar plate: a prospective analysis. Foot & ankle specialist, 6(3), pp.196-200.
McCarthy, C.L. and Thompson, G.V., 2021. Ultrasound findings of plantar plate tears of the lesser metatarsophalangeal joints. Skeletal Radiology, 50(8), pp.1513-1525.