
A 50-year-old lady was referred to physiotherapy with a recurrence and an increase in left Achilles/heel pain.
The patient recalls a long history of Achilles/heel related pain with no formal diagnosis; nevertheless, she was always able to manage her symptoms by adjusting her running regime. However, over the last six months, she increased her running frequency, and the Achilles/heel pain has significantly worsened.
In terms of a daily pattern, her left Achilles/heel pain is worse in the morning but improves after 5-10 minutes of movement. Past medical history includes the early menopause, but the patient is otherwise fit and well. She is taking non-steroidal anti- inflammatories (NSAIDs) to reduce the pain.
On clinical examination there was obvious swelling around the left Achilles tendon (AT) insertion. The patient had full range of movement (ROM) into plantar flexion and inversion. Her knee to wall measurement (KTW) on the affected side was 9cm, compared to 15cm on the unaffected side. Her KTW ROM was limited on the left due to the Achilles/heel pain.
The patient was not able to perform a single leg (SL) calf raise on the left owing to significant pain. She was able to undertake around 17 SL heel raises on the right.
Palpation of the distal AT, insertion and heel area provoked considerable pain. There was no pain at the AT mid-portion. Palpation around the calcaneus was additionally sore.
The differential diagnosis was insertional Achilles tendinopathy (IAT) plus or minus bony involvement, IAT plus or minus retrocalcaneal bursitis or a calcaneal stress fracture.
A Sonosite M-turbo machine (Figure 1) with a high frequency linear array transducer (6-13MHz) was used to perform the scan, the depth and gain were adjust as required to visualise the individual structures.

The patient was examined in prone with the ankle placed over the end of the bed, to allow movement of the talocrural joint and dynamic assessment of the AT and retrocalcaneal bursae.
The Achilles area and calcaneus were assessed in transverse and longitudinal sections, on both the affected and unaffected side to allow comparison and to aid diagnosis.
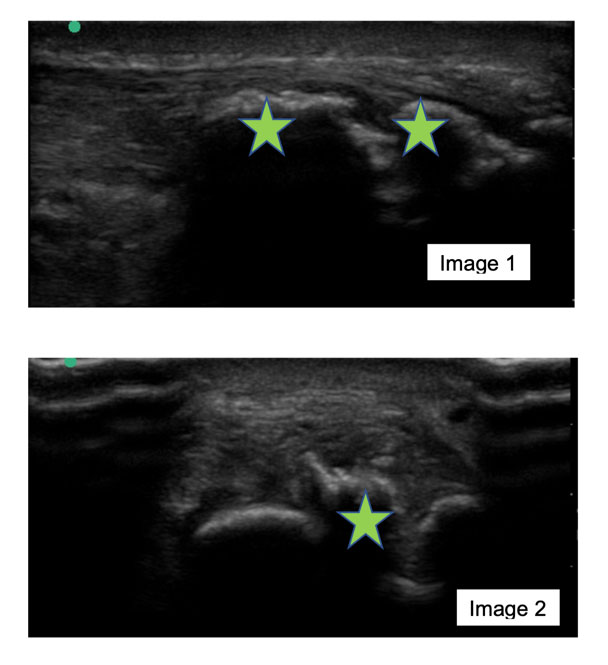
Image 1 is a longitudinal image of the distal AT and calcaneus on the affected side. It demonstrates the footprint of the AT attaching onto the calcaneus, but there is significant bone ossification (green stars) on the calcaneus.
Image 2 is a transverse image of the distal AT and calcaneus, further highlighting the bone ossification. Bony ossification was termed as such, as opposed to a Haglund’s deformity which is a true enlargement of the posterosuperior margin of the calcaneal tuberosity.
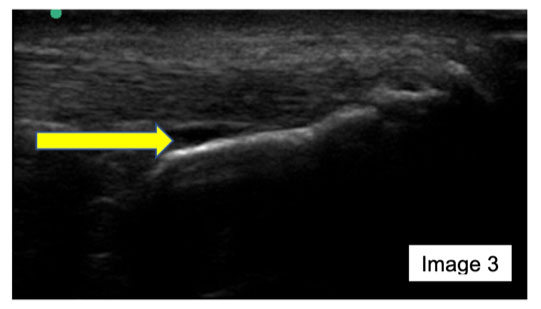
Image 3 is an alternative longitudinal section (compared to image 1) of the distal AT and calcaneus. It demonstrates the footprint of the AT attaching onto the calcaneus, with a mild/moderate retrocalcaneal bursa (yellow arrow).
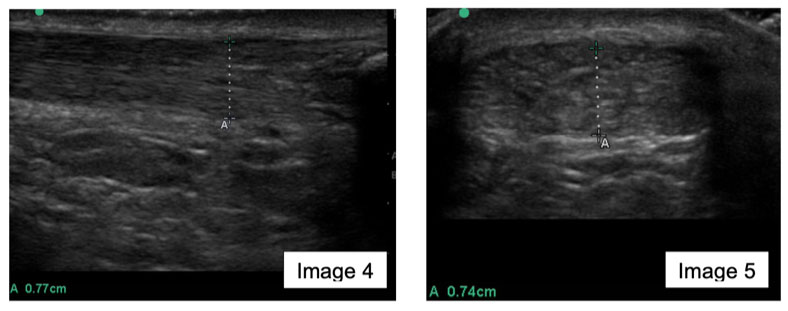
Image 4 is a longitudinal image, with image 5 being transverse of the distal AT, less than 2cm from the insertion. Both highlight tendon thickening, a non-uniform fibril pattern and focal areas of hypoechogenicity. The AT diameter is 7.7mm (image 4) is and 74mm (image 5) respectively.
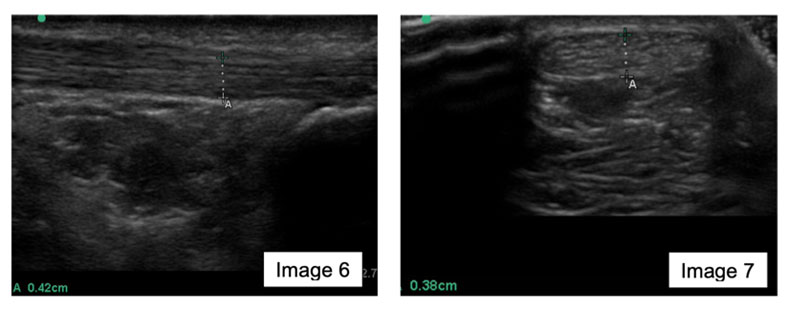
Image 6 is a longitudinal image, image 7 is transverse of the unaffected distal AT, less than 2cm from the insertion. Both images demonstrate, a normal fibrillar pattern with no obvious thickening, with diameter being 42mm (image 6) and 38mm (image 7).
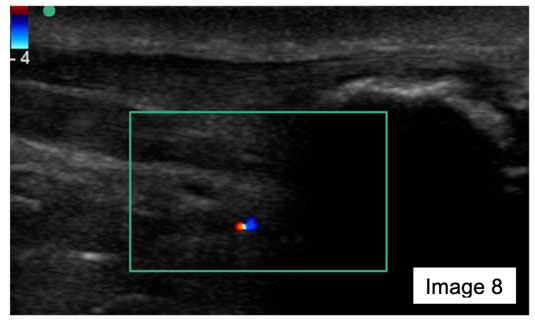
Image 8 is a longitudinal image of the distal AT and calcaneus on the involved side. The image illustrates no neovascularisation of the AT, retrocalcaneal bursa or Kager’s fat pad.
From the diagnostic ultrasound examination, a diagnosis of IAT with bony ossification was confirmed but a calcaneal stress fracture could not be excluded.
Alfredson and Spang (2020) state that IAT often includes pathology in multiple tissues, such as the retrocalcaneal bursa and superficial Achilles bursa, calcaneus, and tendon tissue, and the source of pain can be difficult to diagnose, with intra-tendinous bone formation especially difficult to treat.
Furthermore, IAT is characterised by pain at the posterior heel and appears to serve as a universal term for a large variety of underlying pathologies (Alfredson and Spang, 1998).
The aetiology of IAT is known to be multi-factorial, with several extrinsic and intrinsic factors such as age, obesity, poor training habits and bony spur formation/calcification being some, (Sayana and Maffulli, 2005) along with many other factors.
Specifically, Chimenti et al. (2016) highlighted that patients with IAT used greater end- range dorsiflexion, less plantarflexion, and lower peak ankle plantar flexor power than controls. Patients with greater IAT symptoms also demonstrated decreased use of the ankle plantar flexors during function.
Approximately 6% of the general population reports AT pain during their lifetime (Chimenti et al., 2017), and of these patients, roughly one-third will have IAT (Nicholson et al., 2007).
Zellers et al. (2019) acknowledged diagnostic ultrasound maybe useful in assisting with the differential diagnosis of IAT, helping clinicians identity and confirm treatment targets.
Tendon
With respective to AT pathology, Kahn et al. (2003) demonstrated the accuracy of ultrasound in a study of 85 normal and pathology Achilles tendons (midportion and insertional), reporting that ultrasound identified abnormal morphology in 37 of the 57 (65%) symptomatic tendons and normal morphology in 19 of the 28 (68%) asymptomatic tendons. Hence ultrasound demonstrated a sensitivity of 80% and specificity of 49% in diagnosing tendinopathy compared with the clinical assessment.
From the patient images (image 4 and 5) the main findings were a tendon thickness of greater than 6mm. Soila et al. (1999) reported the average size of the tendon to be 5.2 mm at 32 mm above the calcaneal corner on axial images, while anything more than 6mm in IAT was considered abnormal. This description and definition very much reflected the images 4 and 5 in this patient’s assessment.
Additionally, images 4 and 5 demonstrated a non-uniform fibrillar pattern and focal areas of hypoechogenicity. Klauser et al. (2013) defined these abnormal ultrasound tendon findings as tendon degeneration, with Chimenti et al. (2017) concluding that degenerative changes within the Achilles insertion is the hallmark of IAT.
The use of colour and power Doppler in ultrasound has allowed examination of blood flow and vascularity within and surrounding the Achilles tendon. Zanetti et al. (2003) demonstrated that the presence of neovascularization is a relatively specific sign for a clinically painful tendon.
Yang et al.’s (2010) systematic review identified that many studies have reported neovascularization close to the widest part of the tendinopathy, adding however that the exact correlation between the colour and power Doppler findings and clinical severity remain unclear.
Image 8, in this assessment revealed minimal neovascularization in either the AT, retrocalcaneal bursa or fat pad, possibly indicating that currently none of these were the main symptom generator. The lack of neovascularization around these structures, may give an indication to treatment strategies, which target other structures, such as the bone.
It is important, however, to consider ultrasound as only one component of the assessment, not a stand-alone result. Studies have demonstrated abnormal findings in the AT of 3.8% of asymptomatic individuals in a general population (Joseph et al., 2012).
Furthermore, Sunding et al. (2016) noted that studies in tendon research often refer to tendon thickness, structural changes, and/or the amount of neovascularisation. The reliability of these measurements and the qualitative evaluations are seldom reported, consequently highlighting an area for further research.
Bursae
Correlating this patient’s images with the provisional diagnosis of IAT, the likelihood of bursal pathology was high. Bleakney and White (2005) recognised the advantages of ultrasound in identifying the subcutaneous calcaneal bursa & retrocalcaneal bursa, both located close to the insertion of the AT onto the calcaneus.
After dynamic ankle assessment and likely “milking’ of the retrocalcaneal bursa, image 3 demonstrates a mild/moderate retrocalcaneal bursitis. The benefit of ultrasound and these images in this assessment indicates the bursa has pathology and that it could be source of the patient’s symptoms, likely directing the most appropriate management strategies.
Olivieri et al. (1998), using MRI as the gold standard, found that while US had a high specificity of 100%, it surprisingly had low sensitivity of 50% for diagnosing bursitis.
Nevertheless, the retrocalcaneal bursa can be commonly seen in asymptomatic subjects and varies considerably in appearance and size (Mathieson et al., 1988), so its presence must be treated with restraint.
Conversely the subcutaneous calcaneal bursa is not seen in “normal individuals”; typically, with its appearance on an ultrasound normally only present after an injury or from an inflammatory condition (Frey, 1982). Thus meaning that any positive sighting of the subcutaneous calcaneal bursa needs to be considered significant. In this patient using the stand-off technique, none of the images demonstrated subcutaneous calcaneal bursa pathology.
Bone
The final structure identified on the patient images (images 1 and 2) was bone abnormality on the calcaneus.
With respect to anatomy and pathology, there is ongoing debate about the terminology and meaning of Haglund’s deformity, bone ossification and Achilles insertional calcific tendinosis (AICT) along with various other terms.
Chimenti et al. (2017) concluded that the effect of calcaneal shape on IAT symptoms has not been well supported from the research. It has been speculated that a Haglund’s deformity may theoretically compress against the AT and the retrocalcaneal bursa, which can contribute to IAT symptoms (Shibuya et al., 2012).
Gambart et al. (2021) explains the idea of AICT is a calcification of the distal AT. Pain is gradual onset, in middle age, and typically presents with tenderness to palpation at the insertion along with palpable calcification. Additionally, Gambart et al. (2021) stated Haglund’s deformity patients are young and active. This patient is very much in the former group.
However, in this patient’s images (1 and 2) there seemed to be more bone ossification as opposed to a true Haglund’s deformity or insertional calcific tendinosis.With respect to IAT, ultrasound imaging is found to be as good if not superior to MRI in identifying the presence of bony defects such as a Haglund’s deformity (Chimenti et al., 2016). Van Dijk et al. (2011) also supported the use of ultrasound to visualise the bony abnormalities associated with Haglund’s deformity and calcification within the Achilles tendon.
Utilising other imaging modalities in IAT
Studies have validated ultrasound imaging against x-ray (Chimenti et al., 2016) and MRI (Bullock et al., 2017) for identification of tendon pathology and bony changes at the tendon-bone interface.
Plain x-rays in suspected IAT patients are less utilised overall but are cost effective and efficient in highlighting soft tissue swelling, calcium deposits near the tendon insertion, and a Haglund’s deformity (Nicholson et al., 2007; Bulstra et al., 2015).
MRI is highly sensitive in identifying any abnormalities in patients with posterior heel pain (Nicholson et al, 2007). This would assist in ruling out a possible stress fracture which was initially part of the differential diagnosis and cannot be excluded with ultrasound.
The study by Kahn et al. (2003), which demonstrated the sensitivity and specificity of ultrasound, also looked at MRI in diagnosing tendinopathy, resulting in very comparable results in sensitivity of 90% and specificity of 50%.Solia et al. (1999) assessed 100 ATs on MRI and described that 15% of asymptomatic patients had fluid collections in the retrocalcaneal space and 45% of asymptomatic AT had stripes of increased signal intensity. They additionally noted from all MRIs that the AT insertion “area” had the most notable variations.
In general, the limitations of ultrasound are that there is a limited field of view with linear transducers and a decrease in penetration (Sunding et al., 2016), while another drawback is that it is user dependent, with the learning curve being long and time- consuming (Jacobson, 2002).
Overall, compared to MRI, ultrasound is fast, can be done at the bedside, is reproducible and remains a highly sensitive test for early diagnosis of enthesopathy, generally costing less than MRI (Kamal et al, 2003).
Management options for insertional Achilles tendinopathy
There are various management options available for IAT, with initial management including rest and NSAID medication (Andres and Murrell, 2008).
The disagreement with this approach is that studies demonstrate little, or no inflammation is present in tendons exposed to overuse (Khan et al.,1999).
Consequently, modalities aimed at reducing inflammation have had limited success in chronic painful tendons (Andres and Murrell, 2008).
If a patient’s IAT symptoms persist, a large body of research has advocated physiotherapy rehabilitation. High-quality evidence supports tendon loading for mid portion Achilles tendinopathy (Alfredson et al., 1998; Beyer et al., 2015) with patients overall experiencing less tendon pain and improved function. This principle of mid portion AT loading has been extrapolated to IAT, with less success and requiring subtle modifications in technique.
The muscle contraction type and magnitude of load in tendon management has been hugely investigated, with isometric, eccentric only, and combined concentric/eccentric contractions being used in various combinations with low, medium, and heavy load. Bohm et al. (2015) concluded that for best tendinopathy management, high loading intensities are more effective,compared to low intensities, at inducing adaptive responses, while the type of muscle contraction seems irrelevant.
However, in relation to IAT particularly, Fahlstrom et al. (2003) demonstrated loading technique was critically important. In a group with chronic IAT, eccentric loading over the edge of the step results were not as good as in the group with mid-portion Achilles tendinopathy. The IAT eccentric training gave good results in only 10 out of 31 tendons, with just 32% of patients returning to their previous activity levels. In the other 21 tendons (68%) in the IAT loading over a step, the results were poor, and patients could not return to previous activity levels.
Fahlstrom et al. (2003) speculated the negative effects of eccentric loading over a step for IAT could possibly be explained by the fact that the calcaneal bone might be impinging on a chronically inflamed bursa and/or tendon, during increased angles of dorsiflexion.
This theory was supported by Jonsson et al. (2008) who produced very strong evidence for a modification of “traditional” loading for IAT by avoiding loading over the edge of a step.
27 patients, with a total of 34 tendons diagnosed with IAT, were prescribed a 12-week loading program but only to floor (i.e., not over a step). Following the loading, 18 patients (67%) were satisfied with their outcome and got back to their previous activity while 9 patients (33%) were not satisfied with the treatment but, of note, their pain was significantly reduced.
If this IAT patient fails to respond to a loading programme, the next management option which can be utilised is extracorporeal shockwave therapy (ESWT).
Rompe et al. (2008) compared ESWT (n=25) to eccentric loading (n=25), and found statistically significant improvement for outcomes of pain, activity, and function at 4- month follow-up in the EWST group.
Furthermore, Furia (2005), study consisting of 68 patients, compared ESWT with a control group who received conservative treatment (n=34). Furia (2005) reported the mean visual analogue scale (VAS) for pain for the ESWT group was statistically better at 1, 3, and 12 months after treatment compared with the control group. The percentages of excellent or good results at 12 months were 82.9% and 39.4% in the ESWT and control group respectively. Consequently, it was concluded that ESWT is more effective than physiotherapy/rehabilitation alone for IAT.
The above studies were supported by the National Institute for Health and Care Excellence (NICE, 2016) who advocated the use of ESWT for IAT from their systematic review and meta-analysis on ESWT (n=633).
In addition, NICE (2016) added that there were no major safety concerns with ESWT, but it should only be employed if extra care is taken to explain the risks of treatment. All patients should be screened for appropriate contraindications and precautions prior to ESWT treatment.
If IAT symptoms are still ongoing the next step in this patient’s management, would include more invasive options of injections or surgery.
Alfredson and Spang (2020), Boone et al. (2021) and Solan & Davies (2007) advocated the use of an ultrasound-guided corticosteroid injection (CSI) into the bursa where symptoms of retrocalcaneal bursitis predominate and persist. This was potentially indicated from image 3 during the ultrasound assessment.
Boone et al. (2021), in a study of 218 injections, demonstrated that image-guided retrocalcaneal bursa CSI produced a significant short-term decrease in pain score in the majority (63%) of patients.
The number of studies looking at utilisation of injections in managing IAT is significantly small, consequently, all results promoting injections need to be considered with caution (Wiegerinck et al., 2013).
Surgical procedures for the management of IAT vary hugely. Open debridement and decompression, endoscopic/minimally invasive procedures, gastrocnemius recession, percutaneous procedures, and flexor hallucis longus (FHL) tendon transfer have all been researched and utilised (Chimenti et al., 2017).
Chimenti et al. (2017) summarised that as a technique open debridement has numerous studies reporting high postoperative function and patient satisfaction. Decompression & endoscopic/minimally invasive procedures supposedly may have fewer wound-healing complications, but there is currently insufficient evidence on outcomes. Chimenti et al. (2017) further concluded gastrocnemius recession, percutaneous procedures, and FHL tendon transfers at this stage have limited efficacy and research to advocate their use in managing IAT.
More recently, Alfredson and Spang (2020) have demonstrated the idea of retrocalcaneal bursitis being the main pain source and generator, proposing that this is the main structure that needs to be treated to relieve symptoms in IAT. Alfredson and Spang’s (2020) surgical study produced positive results, with 12/15 tendons, satisfied with the result of a retrocalcaneal bursitis excision and 13/15 tendons getting back to their previous sport and recreational activities at 21 months.
A 50-year-old lady with acute on chronic Achilles/heel pain, with no formal diagnosis, reported an increase in Achilles/heel pain with running. Over a 24-hour period her Achilles/heel pain was worse in the morning. The pertinent findings from her examination were swelling around the AT insertion, while palpation of the distal AT, AT insertion and heel area provoked considerable pain. There was no pain at the AT mid-portion.
Reviewing and analysing the literature, there is a large body of evidence, which supports the use of diagnostic ultrasound in identifying the structures and any abnormality in their characteristics, location and size which could be implicated in IAT. Ultrasound overall is very comparable to MRI in sensitivity and specificity when assessing possible IAT pathology.
Importantly diagnostic ultrasound aids clinicians managing IAT patients allowing them to differentiate between bursa, bone, tendon, and fat pad, achieving a more specific diagnosis and treatment plan. The absence of an ultrasound examination could lead to a gap in identifying specific pathology in these structures potentially leading to sub- optimal management of this patient.
In helping ascertain the patient’s diagnosis, diagnostic ultrasound is a safe, efficient, and cost-effective imaging modality.
Alfredson, H., Pietilä, T., Jonsson, P. and Lorentzon, R., 1998. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. The American journal of sports medicine, 26(3), pp.360-366.
Alfredson, H. and Spang, C., 2018. Clinical presentation and surgical management of chronic Achilles tendon disorders—A retrospective observation on a set of consecutive patients being operated by the same orthopaedic surgeon. Foot and Ankle Surgery, 24(6), pp.490-494.
Alfredson, H. and Spang, C., 2020. Surgical treatment of insertional Achilles tendinopathy: results after removal of the subcutaneous bursa alone—a case series. BMJ open sport & exercise medicine, 6(1), p.e000769.
Andres, B.M. and Murrell, G.A., 2008. Treatment of tendinopathy: what works, what does not, and what is on the horizon.Clinical orthopaedics and related research, 466(7), pp.1539-1554.
Bohm, S., Mersmann, F. and Arampatzis, A., 2015. Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports medicine-open, 1(1), pp.1-18.
Boone, S.L., Uzor, R., Walter, E., Elsinger, E., Catanese, D., Ye, K. and Goldberg- Stein, S., 2021. Safety and efficacy of image-guided retrocalcaneal bursa corticosteroid injection for the treatment of retrocalcaneal bursitis. Skeletal Radiology, pp.1-12.
Bullock, M.J., Mourelatos, J. and Mar, A., 2017. Achilles impingement tendinopathy on magnetic resonance imaging. The Journal of Foot and Ankle Surgery, 56(3), pp.555-563
Beyer, R., Kongsgaard, M., Hougs Kjær, B., Øhlenschlæger, T., Kjær, M. and Magnusson, S.P., 2015. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. The American journal of sports medicine, 43(7), pp.1704-1711.
Chimenti, R.L., Flemister, A.S., Tome, J., McMahon, J.M. and Houck, J.R., 2016. Patients with insertional Achilles tendinopathy exhibit differences in ankle biomechanics as opposed to strength and range of motion. Journal of orthopaedic & sports physical therapy, 46(12), pp.1051-1060.
Chimenti, R.L., Chimenti, P.C., Buckley, M.R., Houck, J.R. and Flemister, A.S., 2016. Utility of ultrasound for imaging osteophytes in patients with insertional Achilles tendinopathy. Archives of physical medicine and rehabilitation, 97(7), pp.1206-1209.
Chimenti, R.L., Cychosz, C.C., Hall, M.M. and Phisitkul, P., 2017. Current concepts review update: insertional Achilles tendinopathy. Foot & ankle international, 38(10), pp.1160-1169.
Furia, J.P., 2005. Extracorporeal shockwave therapy in the treatment of chronic insertional Achilles tendinopathy. Der Orthopade, 34(6), pp.571-578.
Joseph F. M, Anderson M. J, Trojian H. T, et al. Incidence of morphologic changes in asymptomatic Achilles tendons in an active young adult population. J Sport Rehabil. 2012;21(3):249-252.
Jonsson, P., Alfredson, H., Sunding, K., Fahlström, M. and Cook, J., 2008. New regimen for eccentric calf-muscle training in patients with chronic insertional Achilles tendinopathy: results of a pilot study. British journal of sports medicine, 42(9), pp.746- 749
Kamel, M., Eid, H. and Mansour, R., 2003. Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. The Journal of rheumatology, 30(4), pp.774-778.
Khan, K.M., Cook, J.L., Bonar, F., Harcourt, P. and Åstrom, M., 1999. Histopathology of common tendinopathies. Sports medicine, 27(6), pp.393-408.
Khan, K.M., Forster, B.B., Robinson, J., Cheong, Y., Louis, L., Maclean, L., and Taunton, J.E., 2003. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. British journal of sports medicine, 37(2), pp.149-153.
Mathieson, J.R., Connell, D.G., Cooperberg, P.L. and Lloyd-Smith, D.R., 1988. Sonography of the Achilles tendon and adjacent bursae. American Journal of Roentgenology, 151(1), pp.127-131.
National Institute for Health and Care Excellence (2016) Extracorporeal shockwave therapy for Achilles tendinopathy (IPG571)
Nicholson, C.W., Berlet, G.C. and Lee, T.H., 2007. Prediction of the success of nonoperative treatment of insertional Achilles tendinosis based on MRI. Foot & ankle international, 28(4), pp.472-477.
Olivieri, I., Barozzi, L., Padula, A., De Matteis, M., Pierro, A., Cantini, F., Salvarani, C. and Pavlica, P., 1998. Retrocalcaneal bursitis in spondyloarthropathy: assessment by ultrasonography and magnetic resonance imaging. The journal of Rheumatology, 25(7), pp.1352-1357
Rompe, J.D., Furia, J. and Maffulli, N., 2008. Eccentric loading compared with shock wave treatment for chronic insertional achilles tendinopathy: a randomized, controlled trial. JBJS, 90(1), pp.52-61.
Sayana, M.K. and Maffulli, N., 2005. Insertional achilles tendinopathy. Foot and ankle clinics, 10(2), pp.309-320.
Soila, K., Karjalainen, P.T., Aronen, H.J., Pihlajamäki, H.K. and Tirman, P.J., 1999. High-resolution MR imaging of the asymptomatic Achilles tendon: new observations. AJR. American journal of roentgenology, 173(2), pp.323-328.
Solan, M. and Davies, M., 2007. Management of insertional tendinopathy of the Achilles tendon. Foot and ankle clinics, 12(4), pp.597-615.
Wiegerinck, J.I., Kerkhoffs, G.M., Van Sterkenburg, M.N., Sierevelt, I.N. and Van Dijk, C.N., 2013. Treatment for insertional Achilles tendinopathy: a systematic review. Knee Surgery, Sports Traumatology, Arthroscopy, 21(6), pp.1345-1355.
Yang, X., Pugh, N.D., Coleman, D.P. and Nokes, L.D.M., 2010. Are Doppler studies a useful method of assessing neovascularization in human Achilles tendinopathy? A systematic review and suggestions for optimizing machine settings. Journal of medical engineering & technology, 34(7-8), pp.365-372.
Zanetti, M., Metzdorf, A., Kundert, H.P., Zollinger, H., Vienne, P., Seifert, B. and Hodler, J., 2003. Achilles tendons: clinical relevance of neovascularization diagnosed with power Doppler US. Radiology, 227(2), pp.556-560.
Zellers, J.A., Bley, B.C., Pohlig, R.T., Alghamdi, N.H. and Silbernagel, K.G., 2019. Frequency of pathology on diagnostic ultrasound and relationship to patient demographics in individuals with insertional Achilles tendinopathy. International journal of sports physical therapy, 14(5), p.761.